These sorts of polymers have become extremely useful in hospitals for washing soiled linen and hospital clothing. When linen is replaced from beds in hospitals there is an issue for the cleaners/nurses because they have to touch the linen and replace it whilst putting the linen out of patient contact due to risks of infection or illness. It then needs to be sent down to the washing rooms to be cleaned, but how can you stop so many people coming into contact with it?
So, hospital laundry bags are now made out of dissolvable polymers; the cleaner/nurse can put the soiled clothes/linen into a laundry bag straight away, put it in a big trolley bin and then close it, thus protecting the patients from infection. When the trolley gets to the laundry room the bags can be removed, thrown straight into the washing machine and dissolved, coming out with clean linen. This means that anyone who comes into contact with the bag has a much reduced risk of infection.
The plastic used in the bags is "poly(ethenol), made from another plastic, poly(ethyl ethanoate), by the process of ester exchange" (http://www.4college.co.uk/as/poly/Dissolve.php).
Polymers are made up of hundreds of monomers, assembled by repeating units, but for the conversion of monomers into repeating units there are double bonds that must be broken to join the units together in a chain. For example, below left (http://tinyurl.com/cry9r5l) is an image of the repeating unit in poly(ethenol) (n represents a huge number) but the monomer (that produces the repeating unit) is ethanal, an aldehyde containing with carbonyl functional group or more specifically ethenol (below right; http://tinyurl.com/cry9r5l).
The mechanism of turning the ethanal into the repeating unit and thus the polymer poly(ethenol) is a complicated one which involves turning ethanal (above) into ethenol (above) which as can be observed above is "unstable; then made (into poly(ethenol)) though ester exchange rather than polymerisation" (http://tinyurl.com/cry9r5l). Polymerisation is the usual mechanism for making polymers.
The reason why the polymer is able to dissolve in water is due to the hydrogen bonding present in water and poly(ethenol); which occurs when hydrogen is bonded covalently to a highly electronegative element compared to itself, such as Oxygen, and also, when Hydrogen has a lone pair to align with such as one of the two lone pairs on the Oxygen atom in water (as below-http://tinyurl.com/bl3rjqr).

So the "-OH group on poly(ethenol) can interact with the -OH group in water" (http://tinyurl.com/cry9r5l), form hydrogen bonds, and therefore water dissolves poly(ethenol). But as can be observed in the table at the bottom of (http://tinyurl.com/cry9r5l), the solubility of the polymer depends on "the percentage of ester groups which have been removed". But all of the figures show that the polymer is highly soluble in water.
Another use of the dissolvable polymer is in "surgical stitching; this means that the stitches don’t have to be removed" (http://tinyurl.com/cry9r5l).
So, hospital laundry bags are now made out of dissolvable polymers; the cleaner/nurse can put the soiled clothes/linen into a laundry bag straight away, put it in a big trolley bin and then close it, thus protecting the patients from infection. When the trolley gets to the laundry room the bags can be removed, thrown straight into the washing machine and dissolved, coming out with clean linen. This means that anyone who comes into contact with the bag has a much reduced risk of infection.
The plastic used in the bags is "poly(ethenol), made from another plastic, poly(ethyl ethanoate), by the process of ester exchange" (http://www.4college.co.uk/as/poly/Dissolve.php).
Polymers are made up of hundreds of monomers, assembled by repeating units, but for the conversion of monomers into repeating units there are double bonds that must be broken to join the units together in a chain. For example, below left (http://tinyurl.com/cry9r5l) is an image of the repeating unit in poly(ethenol) (n represents a huge number) but the monomer (that produces the repeating unit) is ethanal, an aldehyde containing with carbonyl functional group or more specifically ethenol (below right; http://tinyurl.com/cry9r5l).
The mechanism of turning the ethanal into the repeating unit and thus the polymer poly(ethenol) is a complicated one which involves turning ethanal (above) into ethenol (above) which as can be observed above is "unstable; then made (into poly(ethenol)) though ester exchange rather than polymerisation" (http://tinyurl.com/cry9r5l). Polymerisation is the usual mechanism for making polymers.
The reason why the polymer is able to dissolve in water is due to the hydrogen bonding present in water and poly(ethenol); which occurs when hydrogen is bonded covalently to a highly electronegative element compared to itself, such as Oxygen, and also, when Hydrogen has a lone pair to align with such as one of the two lone pairs on the Oxygen atom in water (as below-http://tinyurl.com/bl3rjqr).

So the "-OH group on poly(ethenol) can interact with the -OH group in water" (http://tinyurl.com/cry9r5l), form hydrogen bonds, and therefore water dissolves poly(ethenol). But as can be observed in the table at the bottom of (http://tinyurl.com/cry9r5l), the solubility of the polymer depends on "the percentage of ester groups which have been removed". But all of the figures show that the polymer is highly soluble in water.
Another use of the dissolvable polymer is in "surgical stitching; this means that the stitches don’t have to be removed" (http://tinyurl.com/cry9r5l).

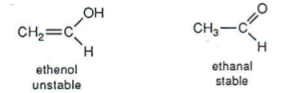
No comments:
Post a Comment